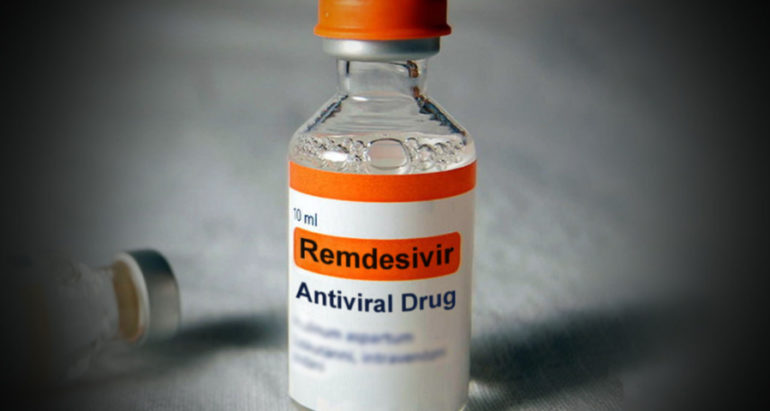
A landmark agreement between Cipla South Africa and Gilead Sciences Inc has led to a manufacturing and distribution license that will have the company producing an effective COVID-19 antiviral medicine locally.
Based upon a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) the Emergency Use Authorization (EUA) for remdesivir was first issued by the United States Food and Drug Administration (FDA) in May 2020. The NIAID study showed that the drug reduced recovery times for hospitalised patients with COVID-19.
The potential benefits of remdesivir outweigh the known risks and the FDA granted the emergency use due to no alternative treatment being available for coronavirus.
Countries such as Japan, India and Singapore also approved the use of the drug and South Africa is among 127 countries that Cipla is now manufacturing the drug for.
The South African Health Products Regulatory Authority (SAHPRA) has also given the drug the go-ahead.
According to Cipla SA CEO Paul Miller, the company’s focus is ensuring patients have an affordable medication to rely on during the pandemic.
“As part of our ethos of Caring for Life, Cipla always aims to ensure that everyone has access to life-saving medication. In much the same way as Cipla pioneered affordable medication during the height of the HIV crisis about two decades ago and helped to save the lives of millions of people, we’re committed to help find a solution in the fight against this unprecedented global pandemic,”says Miller.
In line with their aims, Cipla will ensure remdesivir will be available at an affordable price. The company began manufacturing early in August 2020 and will continue to engage with SAHPRA to ensure access for all.
Picture: Cipla